|
|
Sulfuric acid recovery equipment for Aluminum anodizing process or called sulfuric acid purification for aluminum anodizing process
is a special system using for Aluminum anodizing. It can recover and purify extra Aluminum ions generated during
anodizing process to
maintain Aluminum ion concentration in sulfuric acid bath, so the quality of anodized film can be controlled.
WHAT SULFURIC ACID RECOVERY EQUIPMENT CAN DO FOR YOU? | |
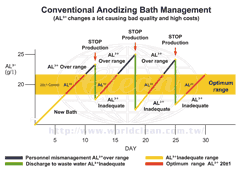
Conventional Anodizing Bath Management (Click for bigger image) |
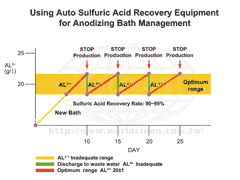
Using Sulfuric Acid Recovery Equipment for Anodizing Bath Management (Click for bigger image) |
Aluminum profiles are widely used in daily life, such as windows and doors, notebook computer, mobile phones … etc. Most of them are based on MIL 8625F Type II class 1 and class2 specification, using oxidation method (anodizing) in sulfuric acid.
Production management parameters in anodizing bath:
- Sulfuric Acid: 100 ~ 250 g / l
- AL3+ Ion: 10 ~ 20 g / l
- Temperature: 18 ~ 25 ℃
- Current Density: 1.0 ~ 2.0 A/dm2
- Oxide film: 7 ~ 20μm
Every anodizing engineer knows these parameters as basic knowledge. Look into the chemical reaction:
- 2H + +2 e- → H2 ↑
- 4OH - 4e ˉ → 2H2O + O2 ↑ (1-2)
- 2Al3 + 3O2 → Al2O3 + exothermic reaction
- Al2O3 + 3H2SO4 → Al2(SO4)3 + 3H2O
When Aluminum is being electrified, a dense layer formed on the surface. Then, dense layer becomes porous layer. The thickness of porous layer grows as electrolysis time increases. When the electrolysis time increases even more, upper portion of oxide film, formed earlier during electrolysis, being soaked in electrolyte for long time, porous layer becomes thinner because pore dissolves. Because the soaking time of the central part of the oxide layer in electrolyte is shorter, the pore wall dissolves less and forming outer small and inverted cone-shaped pores. The electrolysis time continues to increase making walls of the upper pores dissolve into needle-shaped. As the electrolysis time increases, upper parts disappear due to dissolution and near tip parts form needle-shaped again. The lower parts grow and the upper parts dissolve again and again. When oxide film growth rate equals to dissolution rate, the thickness of the oxide film will not be increased even if the electrolysis time increases.
GENERAL ANODIZING PROBLEMS CAUSING UNSTABLE QUALITY:
- For each batch of production, there is differential in the same color. The color difference is bigger for different shape of profile.
- Every plant has two or more anodizing tanks. Appling the same current density to each anodizing tank and then to the same coloring tank, there is still differential in color for the same coloring voltage, time and temperature.
Most engineers will adjust production management parameters of bath (as above chart). For the general continuous production, the liquid H2SO4 ↓ (consumes) and AL3+ ↑ (increase). Automatic dosing will maintain standard concentration while H2SO4 consumes. However, when it AL3+ ↑ (increase), conventional ways to do are as followings (as above chart).
- Based on calculation of analysis and test result, solution in anodizing tank will be discharged to waste water treatment area (Production must be stopped)
To get rid of 1 g/L AL3+ in 200 g/L and 20 Tons anodizing bath, 20 Kg solution must be discharged to maintain at 180 g/L concentration. That is creating burden for waste water treatment, making treatment is not able to work automatically and increasing cost by adding alkaline. Manually adjusting pH of waste water is necessary. - Re-calculating water level and loss of sulfuric acid in order to pour new sulfuric acid down the tank after discharging. (Production must be stopped)
AL3+ can not guarantee at the lowest point of demand standard after discharging. During mass production, work must be stopped for discharging and adding sulfuric acid as describes above. Even for automatic line, it’s the same. - According to Ferrari's Law, AL3+ affects output of anodizing rectifier, causing control difficulties and power loss. The bath warms up fast, so chiller is often high-load running.
In conclusion, AL3+ is the issue causing increase of production costs, instability of product quality and environmental problem.
By using the Sulfuric Acid Recovery Equipment, Aluminum anodizers benefit:
- ENSURE CONSISTENT ALUMINUM OXIDE FILM FORMATION AND STABLE QUALITY.
- REDUCE ENERGY CONSUMPTION DURING ANODIZING.
- REDUCE ENERGY CONSUMPTION OF CHILLERS.
- REDUCE DELAY OF HEAT EXCHANGER AND INCREASE EFFICIENCY OF HEAT TRANSFER.
- REDUCE HUGE LOADS OF WASTE WATER TREATMENT.
- THE EQUIPMENT IS AUTOMATIC OPERATION, ALMOST NO PERSONNEL NEEDED.
- THE EQUIPMENT IS MADE BY HIGH-STRENGTH STAINLESS STEEL AND CORROSION RESISTANT MATERIALS